
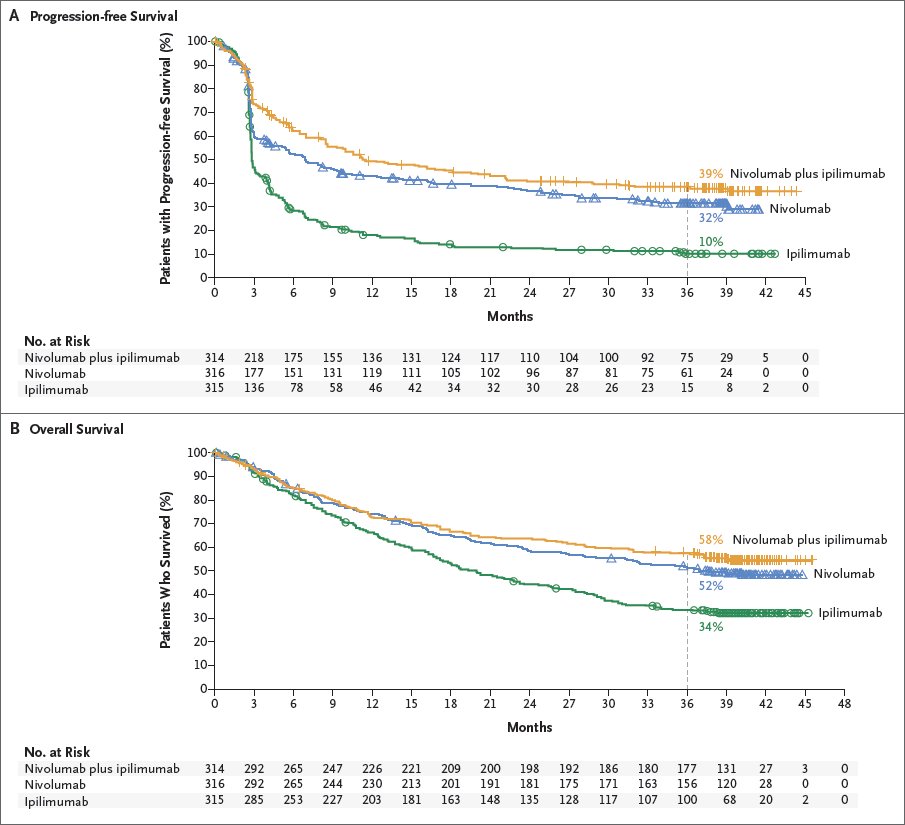
Brahmer is also the codirector of the Cancer Immunology Program and the director of thoracic oncology. Brahmer is codirector of the Upper Aerodigestive Department and a professor of oncology at the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins University Sidney Kimmel Comprehensive Cancer Center in Baltimore, Maryland. In an interview with OncologyLive ®, Brahmer discussed how the data may affect the treatment landscape for NSCLC and the quality-of-life outcomes reported with nivolumab and ipilimumab. “The majority remain treatment free for more than 3 years after treatment discontinuation.” “Regardless of PD-L1 expression level, responses were maintained for greater than 5 years in more than 40% of those who responded ," Brahmer said. At 5 years, the hazard ratio was 0.77 for OS. For those with low PD-L1 expression (< 1%), the 5-year OS rates were 19% vs 7%, respectively, for nivolumab/ipilimumab (n = 187) vs chemotherapy (n = 186). Brahmer, MD, MSc.ĭata presented during the 2022 American Society of Clinical Oncology Annual Meeting showed a 5-year overall survival (OS) rate was 24% with the combination (n = 396) vs 14% with chemotherapy alone (n = 397) among patients with high PD-L1 expression (≥ 1%).
CHECKMATE 9LA CLINICAL STUDY TRIAL
A 5-year analysis of the CheckMate 227 trial (NCT02477826) further supported the use of nivolumab (Opdivo) and ipilimumab (Yervoy) to treat metastatic non–small cell lung cancer (NSCLC) regardless of tumor PD-L1 expression level, according to Julie R.


 0 kommentar(er)
0 kommentar(er)
